Background: Patients (pts) newly diagnosed with FLT3-ITD mutated (m) acute myeloid leukemia (AML) who are ineligible for intensive induction chemotherapy (IC) experience poor outcomes (Konopleva et al. 2022, Clin. Can. Res.). These patients have a median overall survival (OS) of 9.9 months when treated with the standard of care regimen (azacitidine and venetoclax).
Aims: We evaluated the safety and efficacy of the triple combination of Quizartinib (Quiz), Venetoclax (VEN), and decitabine (DAC) in pts with relapsed/refractory (R/R) or newly diagnosed FLT3-ITDm AML.
Methods: The study included a frontline cohort of pts ineligible for IC and an R/R cohort of pts who had received ≤ 5 prior treatments for AML (up to salvage 5). On Day 14, all pts underwent bone marrow (BM) evaluation, and VEN (400 mg/day or equivalent with azole) was discontinued on Day 14 for pts with BM blasts ≤ 5% (or marrow aplasia/hypocellularity). Pts with Day 14 BM blast >5% continued VEN for 21 days during cycle 1, followed by administration from days 1 to 14 in subsequent cycles, with further dose reductions based on count recovery. All pts received 10 days of DAC (20 mg/m2) for induction, followed by 5-day DAC in subsequent cycles. Quiz (30 or 40 mg/day) was continuously administered daily.
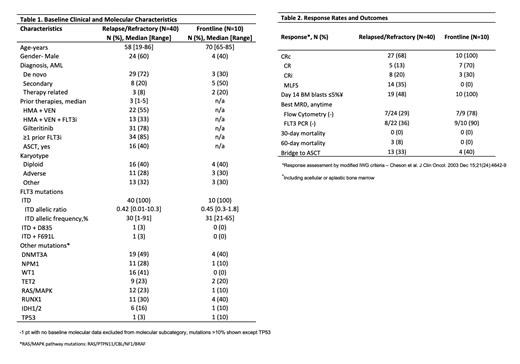
Results: A total of 50 pts were enrolled, including 40 R/R and 10 newly FLT3-ITD mutated AML pts (Table 1). Among the 40 R/R AML pts (median 3 [range 1-5] prior AML therapies), 85% had received ≥1 prior FLT3 inhibitors (FLT3i's), with 78% having prior exposure to gilteritinib, and 40% having undergone prior allogeneic stem cell transplantation (ASCT). The rates of CRc, CR+CRi, measurable residual disease (MRD) negativity by multicolor flow cytometry (MFC, sensitivity 10 -4), and FLT3-PCR (sensitivity 10 -2 - 10 -3) negativity among the 40 R/R pts were 68%, 33%, 29% (7/24), and 36% (8/22), respectively (Table 2). The median number of cycles required to achieve a response was 1 (range 1-2). The 60-day mortality rate was 0%. With a median follow-up of 33 months, the median OS was 7.1 months, and the 1-year OS rate was 22% in this predominantly gilteritinib-exposed R/R cohort. Among the 31/40 prior gilteritinib exposed pts the CRc, CR+CRi, median OS and 1-year survival were 65%, 23%, 6.9 month, and 21%. Among the 9/10 prior gilteritinib naïve pts the CRc, CR+CRi, median OS and 1-year survival were 77%, 66%, 10.3 months and 25%. Among the newly diagnosed AML, all achieved CRc (7 CR, 3 CRi), with 9/10 (90%) and 7/9 (78%) evaluable responders being FLT3-PCR and MFC negative, respectively. All 10 pts achieved BM <5% or aplasia/hypocellularity on C1D14. With a median follow-up of 15 months, the median OS was not reached.
No pts experienced dose-limiting toxicity (DLT) with Quiz 30 mg/day; however, with Quiz 40 mg/day, the first 2 pts treated developed hematologic DLT (grade 4 neutropenia with a <5% cellular bone marrow with no residual AML lasting ≥42 days). Consequently, Quiz 30 mg/day was declared the RP2D for this combination. In the R/R cohort, grade ≥3 non-hematologic toxicities regardless of attribution, occurring in >10% of pts, included pneumonia (73%), neutropenic fever (53%), sepsis (18%), bacteremia (15%), other infections involving the skin (18%), and gastrointestinal tract (13%). The incidence of grade ≥3 non-hematologic toxicities was less frequent in the frontline cohort, with pneumonia (40%), neutropenic fever (50%), sepsis (10%), bacteremia (20%), and no grade 3 QTcF prolongations observed.
The median recovery times for absolute neutrophil count (ANC > 500/μL) and platelet (>50 x 109/L) were 43 days [13-138] and 49 days [21-67] for the R/R cohort, and 40 days [28-56] and 40 days [22-46] for the frontline cohort, respectively.
In the frontline cohort, at the last follow-up, 7 pts were in complete remission (CR), 2 ps died with relapsed AML, and 1 pt died in remission postASCT. Among the 40 pts in the R/R cohort, 9 were still alive (7 in CR, 2 with relapsed disease), while 31 pts died (5 deaths in CR, 26 due to relapsed/refractory leukemia).
Conclusion: The combination of DAC + VEN + Quiz demonstrated activity in heavily pretreated and prior FLT3i-exposed (including 78% with prior gilteritinib exposure) R/R FLT3-ITDm pts, with a CRc rate of 68% and a median OS of 7.1 months. In the frontline setting, all pts achieved CRc with no early mortality, median count recovery of 40 days, and median OS not reached. The study continues to accrue, and updated results will be reported at the meeting.
Disclosures
Yilmaz:Daiichi-Sankyo: Research Funding; Pfizer: Research Funding. DiNardo:BMS: Honoraria; AbbVie/Genentech: Honoraria; Notable Labs: Honoraria; Servier: Honoraria; Astellas: Honoraria; ImmuniOnc: Honoraria; Fogham: Honoraria; Novartis: Honoraria; Takeda: Honoraria; Schrödinger: Consultancy. Kadia:SELLAS Life Sciences Group: Research Funding; Pfizer: Consultancy, Research Funding; Cure: Speakers Bureau; Pulmotect, Inc.: Consultancy, Research Funding; Delta-Fly Pharma, Inc.: Research Funding; Liberum: Consultancy; Amgen, Inc.: Research Funding; Jazz Pharmaceuticals, Pfizer, Pulmotect, Inc, Regeneron Pharmaceuticals, SELLAS Life Sciences Group: Research Funding; Iterion: Research Funding; GenFleet Therapeutics: Research Funding; Ascentage Pharma Group: Research Funding; Sanofi-Aventis: Consultancy; Genzyme: Honoraria; Biologix, Cure, Hikma Pharmaceuticals: Speakers Bureau; AstraZeneca: Research Funding; Hikma Pharmaceuticals: Speakers Bureau; Regeneron Pharmaceuticals: Research Funding; Janssen Research and Development: Research Funding; Glycomimetics: Research Funding; Genentech: Consultancy, Research Funding; Cyclacel: Research Funding; Cellenkos Inc.: Research Funding; Celgene: Research Funding; Astellas Pharma Global Development: Research Funding; Novartis: Consultancy; Servier: Consultancy; Agios: Consultancy; Daiichi Sankyo, Genentech, Inc., Genzyme, Jazz Pharmaceuticals, Liberum, Novartis, Pfizer, PinotBio, Inc, Pulmotect, Inc, Sanofi-Aventis, Servier: Consultancy; AbbVie, Amgen, Inc, Ascentage Pharma Group, Astellas Pharma Global Development, Astex, AstraZeneca, BMS, Celgene, Cellenkos Inc, Cyclacel, Delta-Fly Pharma, Inc, Genentech, Inc., Genfleet, Glycomimetics, Iterion, Janssen Research and Development: Research Funding; Pinotb-Bio: Consultancy; BMS: Consultancy, Research Funding; Astex: Honoraria. Konopleva:AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Clinical Trials Support, Research Funding. Borthakur:Astex Pharmaceuticals, Ryvu, PTC Therapeutics: Research Funding; Pacylex, Novartis, Cytomx, Bio Ascend:: Membership on an entity's Board of Directors or advisory committees; Catamaran Bio, Abbvie, PPD Development, Protagonist Therapeutics, Janssen: Consultancy. Pemmaraju:Protagonist Therapeutics, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees; EUSA Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Magdalen Medical Publishing: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; United States Department of Defense (DOD): Research Funding; Karger Publishers: Other: Licenses; ClearView Healthcare Partners: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Medscape: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Intellisphere: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pacylex: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; OncLive: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Aplastic Anemia & MDS International Foundation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; PharmaEssentia: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ImmunoGen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Physician Education Resource (PER): Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CTI BioPharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Patient Power: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Curio Science: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; CareDx: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CancerNet: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Stemline: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Imedex: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Dava Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ASH Committee on Communications: Other: Leadership; ASCO Cancer.Net Editorial Board: Other: Leadership; National Institute of Health/National Cancer Institute (NIH/NCI): Research Funding; PeerView Institute for Medical Education: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Menarini Group: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Neopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Harborside Press: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Dan's House of Hope: Membership on an entity's Board of Directors or advisory committees; Cimeio Therapeutics AG: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Blueprint: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Aptitude Health: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; HemOnc Times/Oncology Times: Other: Uncompensated; Bristol Myers Squibb Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Short:AstraZeneca: Consultancy; Stemline therapeutics: Research Funding; Amgen: Honoraria; Novartis: Consultancy; Pfizer: Consultancy; Takeda: Consultancy, Research Funding; Astellas: Research Funding. Alvarado Valero:CytomX Therapeutics: Consultancy; Astex: Research Funding; MEI Pharma: Research Funding; Daiichi-Sankyo: Research Funding; Sun Pharma: Consultancy, Research Funding; FibroGen: Research Funding; BerGenBio: Research Funding; Jazz: Research Funding. Maiti:Celgene: Research Funding; Lin BioScience: Research Funding. Masarova:MorphoSys US: Membership on an entity's Board of Directors or advisory committees. Montalban-Bravo:Takeda: Research Funding; Rigel: Research Funding. Loghavi:Caris Diagnostics: Consultancy; Blueprint Medicine: Consultancy; Abbvie: Consultancy; Gerson Lehrman Group: Consultancy; QualWorld: Consultancy; Guidepoint: Consultancy; Recordati/ EUSA Pharma: Consultancy; Daiichi Sankyo: Consultancy; Astellas: Research Funding; Amgen: Research Funding; Abbvie: Current equity holder in publicly-traded company. Jabbour:Adaptive Biotech: Consultancy, Honoraria, Research Funding; Genentech: Consultancy, Honoraria, Research Funding; Hikma Pharmaceuticals: Consultancy, Honoraria, Research Funding; Ascentage Pharma Group: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding. Garcia-Manero:Genentech: Research Funding; Bristol Myers Squibb: Other: Medical writing support, Research Funding; AbbVie: Research Funding. Ravandi:Astellas: Consultancy, Honoraria, Research Funding; Xencor: Research Funding; Prelude: Research Funding; Amgen: Honoraria, Research Funding; Astex/taiho: Membership on an entity's Board of Directors or advisory committees, Research Funding; Syros: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Biomea fusion: Honoraria, Research Funding; Celgene/BMS: Consultancy, Honoraria, Research Funding. Andreeff:Kintor Pharmaceutical: Research Funding; PMV: Research Funding. Daver:ImmunoGen: Consultancy, Research Funding; Gilead: Consultancy, Research Funding; Servier: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Novartis: Consultancy; Shattuck Labs: Consultancy; AROG: Consultancy; AbbVie: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Celgene: Consultancy; Agios: Consultancy; Kite, a Gilead company: Consultancy, Research Funding; Novimmune: Research Funding; Syndax: Consultancy; Pfizer: Consultancy, Research Funding; Glycomimetics: Research Funding; FATE: Research Funding; Trovagene: Research Funding; Jazz: Consultancy; Astellas: Consultancy, Research Funding; Hanmi: Research Funding; Trillium: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Kronos Bio: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal